The term 'molecular editing' was coined by Danishefsky in reference to the synthetic chemist's unique ability to revise specific elements of a natural product's structure in order to modulate properties such as pharmacokinetic behavior or chemical stability. While natural products have been refined through cycles of selection/diversification, they are often suboptimal as orally bioavailable pharmacological agents and, thus, 'editing' particular metabolic or chemical 'soft spots' within a naturally occurring molecular structure can be beneficial to its development as a drug candidate. For example, the complex molecular framework of the antifungal triterpenoid glycoside, enfumafungin, was modified in the course of medicinal chemistry studies to excise undesirable functionality such as a cyclic hemiacetal and C3-O monosaccharide moiety (highlighted below in red and blue, respectively), both of which were found to be either chemically or enzymatically labile. These research efforts culminated in the development of the antifungal clinical candidate SCY-078, the first orally active glucan synthase inhibitor. Satori Pharmaceuticals have recently disclosed results from a similar natural products-based program aimed at the development of novel gamma-secretase modulators as therapeutics for Alzheimer's disease, starting from a triterpenoid glycoside (1) isolated from the black cohosh plant. The plant sterol natural product 1, like enfumafungin, exhibits exquisite and unique bioactivity but contains a few potential metabolic and chemical liabilities, which include a C3-O glycoside (blue) and C24 enol ether (red). Molecular editing through semisynthetic derivatization and medicinal chemistry optimization produced investigational lead compounds such as 2 that display improved potency and central nervous system (CNS) exposure.
The mode of action of 1 and 2 involves modulation of the peptidic site on amyloid precursor protein (APP) where gamma-secretase cleavage occurs. In brief, APP is cleaved at various sites by gamma-secretase to produce different amyloid-beta peptides. The relatively longer amyloid-beta fragments (which are around 42 amino acids long as opposed to 38 or 40) seem to be particularly neurotoxic with regard to initiation of the pathology of the Alzheimer's brain. Therefore, a small molecule that could lower the ratio of longer amyloid fragments relative to the shorter ones could represent a safe and tolerable therapeutic agent for Alzheimer's disease, devoid of the toxicities that arise from an overall blockade of the action of gamma-secretase (which include skin cancer). It should be noted as an aside that the steroidal lactone withanolide A also alters APP processing through an alternate mechanism that is discussed here.
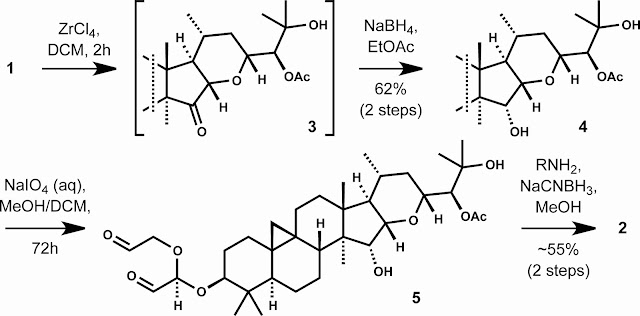
The lead plant sterol derivative 2 is synthesized in four steps from the triterpenoid natural product 1. First, stereoselective reduction of the C16-C17 olefin is achieved by a two step sequence involving zirconium tetrachloride-catalyzed isomerization of the enol ether followed by reduction of the C15 ketone (dr > 95:5). Double oxidative cleavage of the C3-O glycoside by treatment of 4 with sodium periodate then secured a versatile dialdehyde intermediate (5) from which a variety of C3 morpholino derivatives could be obtained by application of a double reductive amination protocol. Derivative 2 effectively lowers levels of amyloid-beta 42 and 38 while preserving amyloid-beta 40 and total amyloid-beta. In addition, 2 exhibits improved CNS penetration relative to 1 and retains an excellent profile in standard transporter, off-target and safety assays. This natural products-based approach represents an exciting entry into gamma-secretase modulation (rather than inhibition) as a potential therapeutic approach to the treatment of Alzheimer's disease.